Learning Outcomes
By the end of this lesson, students should be able to:
i. Explain the concept of reference standards in proton NMR spectroscopy and the role of the tetramethylsilane (TMS) scale.
ii. Describe the utilization of chemical shift values in identifying functional groups and structural motifs in organic compounds.
iii. Interpret coupling constants (J values) and their relationship to the number of bonds between protons and the geometry of the molecule.
iv. Apply their knowledge of standard scales and coupling constants to extract structural information from proton NMR spectra.
v. Appreciate the significance of reference standards and coupling constants in enhancing the understanding of molecular structure through proton NMR spectroscopy.
Introduction
Proton NMR (nuclear magnetic resonance) spectroscopy provides a rich source of information about the structure and connectivity of organic molecules. However, interpreting proton NMR spectra requires a standardized system for measuring and reporting chemical shifts and coupling constants. This lesson delves into the world of standard scales in proton NMR, guiding students through the concepts of the tetramethylsilane (TMS) scale, chemical shift interpretation, and coupling constants.
i. The Tetramethylsilane (TMS) Scale: A Universal Reference
The tetramethylsilane (TMS) scale serves as the universal reference standard for proton NMR spectroscopy. TMS, with its chemically inert and symmetrical structure, exhibits a very high chemical shift and is set as zero ppm. All other proton chemical shifts are reported relative to TMS.
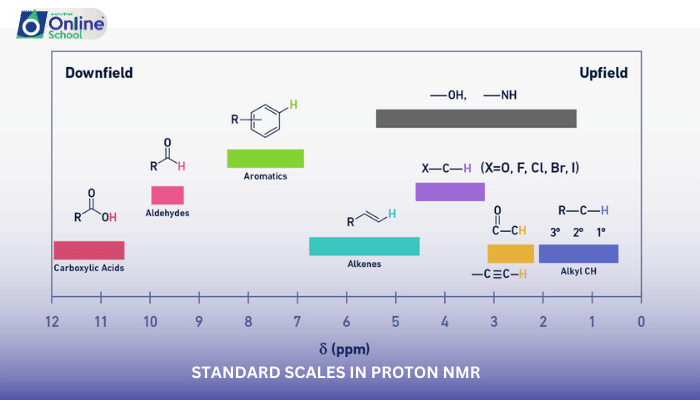
ii. Chemical Shifts: Unraveling Functional Groups
Chemical shifts provide valuable insights into the chemical environment of protons within a molecule. Different functional groups exhibit characteristic chemical shift ranges, allowing chemists to identify functional groups based on their proton resonances in the NMR spectrum. For instance, alkyl protons typically appear in the range of 0-1 ppm, while aromatic protons are found around 6-8 ppm.
iii. Coupling Constants (J Values): A Geometric Fingerprint
Coupling constants, denoted by J values, represent the magnetic interaction between non-equivalent protons. These interactions lead to the splitting of NMR peaks into multiplets, such as doublets, triplets, and quartets. The magnitude of the coupling constant (J value) provides information about the number of bonds between the interacting protons and the geometry of the molecule.
iv. Interpreting Spectra: A Structural Jigsaw Puzzle
By combining chemical shift values and coupling constants, chemists can extract detailed structural information from proton NMR spectra:
Identify Functional Groups: Recognize functional groups based on their characteristic chemical shift ranges.
Determine Connectivity: Analyze splitting patterns to determine the number of bonds between protons and the geometry of the molecule.
Piece Together the Structure: Combine information from chemical shifts, coupling constants, and integration to deduce the overall structure of the molecule.
The use of standard scales, such as the TMS scale, and the interpretation of chemical shifts and coupling constants are essential for extracting structural information from proton NMR spectra. By understanding the relationship between these parameters and the molecular structure, chemists can effectively interpret NMR spectra and unravel the intricate details of organic compounds, gaining a deeper understanding of their properties and reactivity.